English

Company overview
Trade name AMBiS Corp.
Address 2013 Ozato, Nanjyo-City, Okinawa, Japan 901-1202
TEL +81-98-835-8878
FAX +81-98-835-8879
Est. Jun.23 1999
The capital 50,000,000JPY
Representative Director Masanao Kikukawa
Director Sakae Arakaki
Director Masatoshi Nozaki
Director Fuminari Karakawa
Auditor Kyouko Nakayama
Auditor Kameshirou Watanabe

History
Jun.1999 Establishment
Jan.2000 "Biotechnology summit symposium 2000in Okinawa" is held.
Mar.2000 Venture company support region consortium business(NEDO)
"Development of extraction and refinement of high purity of making to handiness large-scale
plasmid" ( Trust Patent JP 547715 US6,773,913B2)
Jan.2002 Business scheme (creation method) recognition from Okinawa Prefecture like research and
development etc.
Mar.2002 Japan Small and Medium Enterprise Corporation new business development
subsidy delivery business
"Development of efficiency production method of material that lies vector
production" (Trust Patent JP2005-052135)
Mar.2002 The Cabinet Office Okinawa General Bureau region creation description
research and development business
Dec.2003 Trust Patent fillings"Body rearrangement of five hetero type amounts vaccine“
Sep.2004 Biotechnology venture company research and development support business
trust from Okinawa Prefecture
It executes it the business for 06 years '05' by "Development of the process of manufacture
with the medicine grade manufacturing device that uses a genetically modified microorganism"
continuance adoption.
Jan.2007 The recognition of "Plan of development in new coordinated business field for a different type
of business" by the head of Okinawa General Bureau
of ..Minister of Health, Labour and Welfare.. the Cabinet Office is received.
Oct.2007 Medicine manufacturing authorization such as medicines made of biology
medicine:The 47th AZ100001 of permission numbers
Jan.2008 Production beginning of vector of GMP conforming plasmid DNA
It is a change of trade name in AMBiS Ltd. from the up-to-date medical
Aug.2008 student thing science laboratory Ltd..
Access
GMP Drug design support enterprise
■ Plasmid production by commissioning quality at equipment of GMP conformity
Medical-supplies manufacturing industry (biologicals) (47th AZ No. 100001) acquisition is carried out, it also has the manufacture institution of GMP conformity, and dealing also with manufacture of an investigational new drug is possible in our company.

■Feature
・It connects for complete closure system sanitary piping.
・Bubble cancellation equipment is installed and it is 70%
or more of cultivation capacity efficiency.
・CIP washing and sterilization, and SIP sterilization are
possible.
・completeness -- operating automatically -- operating
record preservation
・A bacillus, the sanitization at the time of recovery
・Patent JP3547715 US6,773,913B2
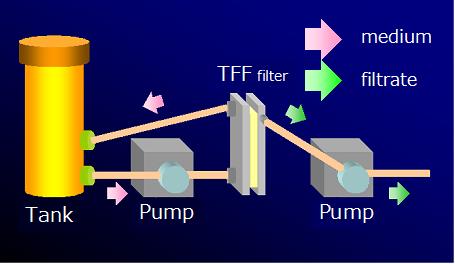
■ Extraction by TFF film use, filtration We enabled concentration of the plasmid which made centrifugal separation operation unnecessary, and buffer exchange by a continuation closing system by introducing a TFF system. A pump is made into the source of power, an undiluted solution is refluxed in a system, liquid is poured in parallel with an emulsion side, and removal of impurities and concentration of a required thing are performed. By selecting various kinds of films into a filter portion, it can respond to various molecule sizes.
The contents of trust
【 Receiving inspection 】
・We keep a recombination plasmid and a genome sequence is checked.
【 Test manufacture 】
・Before real manufacture, culture condition examination and
manufacture examination with a small scale are performed, and check of quantity and
examination of refining conditions are performed.
【 Manufacture 】
・A master cell bank (MCB) and a working cell bank (WCB) are
manufactured by GMP conformity. Moreover, the plasmid DNA refines more than 1 lot 500mg.
【 Quality examination 】
・MCB/WCB Quality examination
(MCB/WCB The example of an examination item )
Transgenics object ・Base sequence analysis ・Andtoxin ・ Purity test・ Soundness test etc
Host ・・・・・・・・Characteristic analysis examination ・ Purity test・Soundness test etc
Transgenics object ・Base sequence analysis ・Andtoxin ・ Remains antibiotic check test・Purity test ・Soundness test etc
【 In addition, service 】
・MCB/WCB and the refined plasmid. DNA will deliver amount of note to Baial acceding to a request.
・Plasmid DNA also hears concentration adjustment according to demand.
・MCB/WCB Storage of WCB is also heard.
*The above-mentioned quality examination item is planning the examination based on advice of
ICH.(International Conference on Harmonisation of Technical Requirements for Registration of
Pharmaceuticals for Human Use.
For details, We determine after arranging.
Genetic engineering experiment support
We are offering various trust services of the genetic engineering experiment, in order to support the increase in efficiency of the research
relevant to genetic engineering.
Cloning
Clone creation which inserted the purpose gene in the vector of hope is
performed.
Plasmid extraction
It corresponds also to Plasmid extraction with the small lot of μg unit.
DNA extraction
It refines by carrying out DNA extraction from a cultivation cell, a plant,
and bacteria (after consulting about other samples).
RNA extraction
It refines by carrying out RNA extraction (after consulting about other
samples) from a cultured cell, a plant, and bacteria.
cDNA extraction
RNA extraction and refining are performed from a cultivation cell, a plant, and bacteria (after consulting about other samples), and cDNA
composition is performed.
Northern blotting
RNA is extracted, and electrophoresis of what was fragmented in specific size by the restriction enzyme is carried out in agarose gel, it dissociates,
it is transferred to a membrane, and the target RNA is detected.
Southern blotting
DNA is extracted, and electrophoresis of what was fragmented in specific size by the restriction enzyme is carried out in agarose gel, it dissociates,
it is transferred to a membrane, and the target DNA is detected.
Protein experiment support
Construction of protein expression vector
The clone that inserts a target gene in the expression vector of hope is made.
*The customer must prepare the expression vector.
Confirmation of expression
It cultures on a small scale after it transforms it to the landlord, and the expression is confirmed.
(SDS-PAGE、 Western 、ELISA、etc…)
Large-scale culture
The high appearance clone obtained because of the expression confirmation is cultured with the large-scale culture device.
Protein refinement
The sample is refined by using various refinement methods like the affinity and the gel filtration, etc.
Patent
■High purity of making to handiness large-scale plasmid extraction and refinement technology
(Patent JP 547715 US6,773,913B2)
Device and method for vector refinement to be enforceable of concentrated operation etc. of culture solution continue in close system and to obtain vector of high purity.
In the vector refinement device, the bacterium including the vector is cultured by the culture solution in the culture tank, it filters by the first TFF filtration film, this culture solution is concentrated, the compound liquid of the culture solution and the buffer liquid is filtered by the TFF filtration film as the buffer tank supplying the buffer liquid, and the culture solution is exchanged for the buffer liquid. The alkali tank supplies the alkaline fluid, the lyses bacterium is passed through the second TFF filtration film, and waste is removed. Because the TFF filtration film is used without using the centrifugal separator to concentrate it, a series of operation is enforceable continue in the close system.
The customer who examines medicine GMP made of the living thing equipment is done the plant engineering design by the above-mentioned license.
■Five hetero amount body(Patent JP2005-052135)
Vaccine that enables production at industrial level by improving production level and refinement efficiency and component vaccine of Japanese encephalitis.
Five hetero type amount body composed of non-uniting monomer that consists of amino acid sequence of monomer of uniting monomer and mucous membrane connectivity protein that consists of fusion protein with amino acid sequence of monomer of amino acid sequence and mucous membrane connectivity protein with immunogenic agents. Five homo type amount body composed of uniting monomer that consists of fusion protein with amino acid sequence of monomer of Japanese encephalitis virus outline protein origin antigen and mucous membrane connectivity protein. These five hetero type amount body or five homo type amount bodies can be used as a vaccine.
It is possible to use it for the usage such as the oral vaccines.

joint research and research trust
■Anti-habu venom homo antibody making research
This research confirms appearance by the cloning and Chinese hamster ovary cell of the gene of the hub poison neutralizing homo antibody to make the anti-habu venom homo antitoxin that a genetically modified technology is used by the trust from an Okinawa Prefecture hygiene environmental laboratory and the danger of the side effect is a little, and does the research that refines the antibody after it cultures it in large quantities.
■Vaccine development for pig of Japanese encephalitis
The guesswork industry is planning the development of the vaccine for the pig of Japanese encephalitis in Kobe University, Ryukyu University, and our company in the following stage "Promotion stage" of "Educational-industrial complex seeds innovation making business actualizing stage (The problem name: verification of the reformative disease prevention for the animal by a protein mixture vaccine and DNA/needle no dispensing method)" in 2006 fiscal year. Our company plans to take charge of manufacturing the plasmid that becomes DNA vaccine in the guesswork industry.
It does a joint research using a research that uses the rearrangement body, a research that needs the large scale culture or the subtropics climate and the research is entrusted.

プラスミドは株式会社AMBiS
〒901-2214宜野湾市我如古2-12-16 ルミエール201号室
TEL 098-988-1503
FAX 098-988-4504
ambis@ambis.co.jp
Copyright(C) 2009 AMBiS Corporation. All Rights Reserved.